BriaCell Reports Complete and Sustained Resolution of Brain Metastasis and Sustained Regression of Orbital Metastasis in “Eye-Bulging” Breast Cancer Patient
- [IMAGES BELOW] Sustained complete resolution of temporal lobe brain metastasis and continued orbital tumor reduction after >18 months of treatment
- “Eye bulging” metastatic breast cancer patient had failed 8 prior regimens, including antibody-drug conjugate (ADC) therapy
-
Patient remains on BriaCell’s Phase 2 study with 29 treatment cycles completed
PHILADELPHIA and VANCOUVER, British Columbia, July 10, 2025 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW, BCTXZ) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company developing novel immunotherapies to transform cancer care today announced updated results from its ongoing Phase 2 study of Bria-IMT™ in combination with check point inhibitor in patients with advanced metastatic breast cancer (MBC).
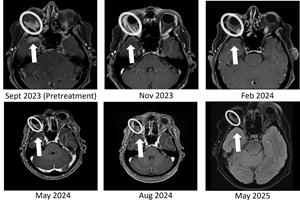
BriaCell is pleased to report the sustained complete resolution of temporal lobe brain metastasis and continued orbital (behind the eye) tumor reduction after > 18 months of treatment in the Phase 2 study. The metastatic tumor initially caused proptosis –a visibly bulging of the eye. As previously reported, the heavily pre-treated MBC patient had demonstrated a complete resolution of a temporal lobe brain metastasis and a significant response in a right orbital metastasis at 8 months, then at 11 months. The patient has now maintained both responses for more than 18 months, with no evidence of brain tumor recurrence and ongoing tumor shrinkage in the orbital lesion.
Figure 1: Bria-IMT treatment resulted in complete resolution of the right temporal lobe lesion and continued regression of the right orbital (behind the eye) tumor. The right temporal lobe lesion is no longer detectable on the images taken at 8 months (May 2024), 11 months (Aug 2024), and 20 months (May 2025).
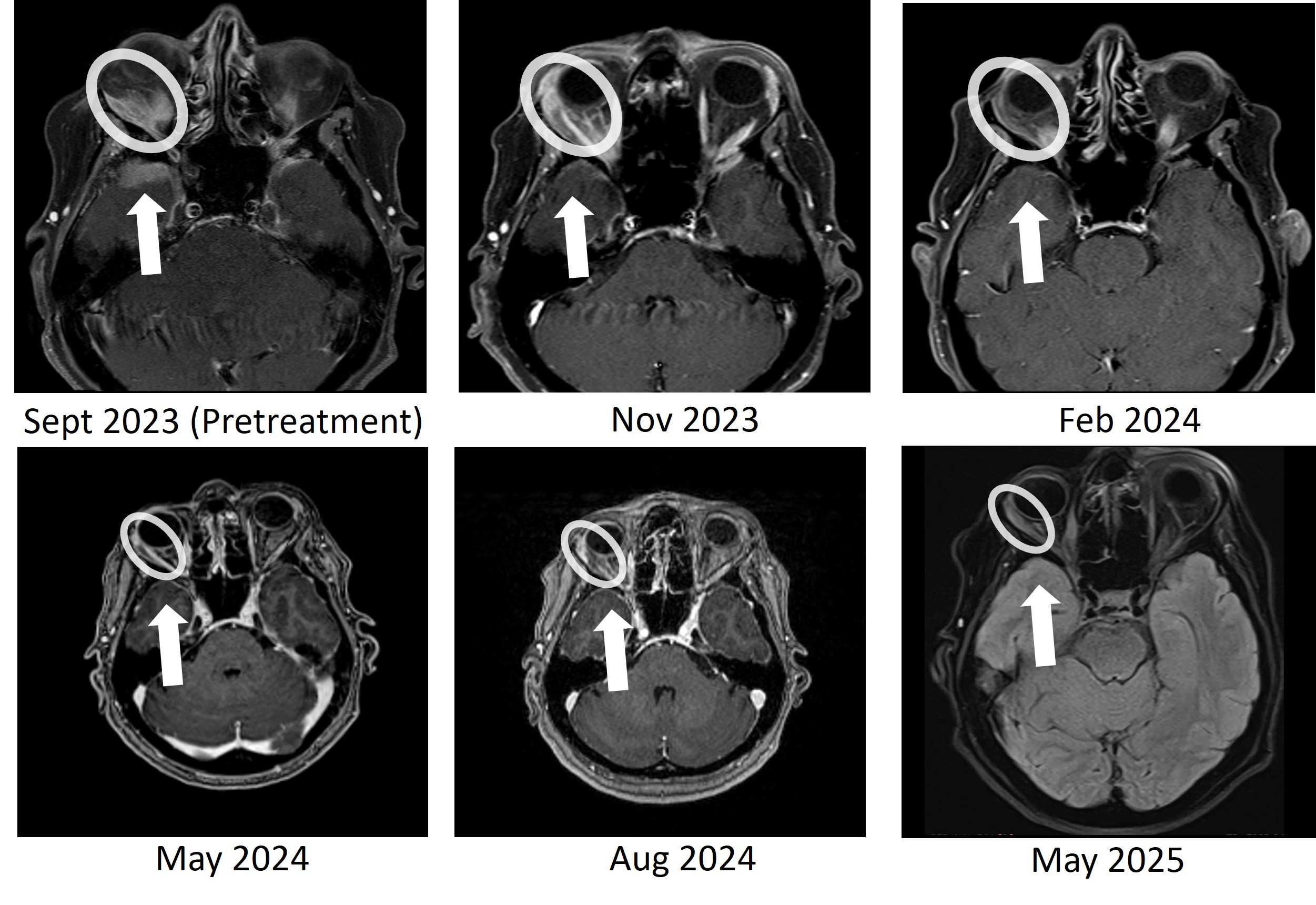
This patient had failed eight prior treatment regimens, including an antibody-drug conjugate (ADC), before initiating therapy with Bria-IMT plus checkpoint inhibition. She has now completed 29 treatment cycles and has been on BriaCell’s Phase 2 study for over 21 months. Serial imaging at 8, 11, and now 20 months have confirmed no detectable disease in the right temporal lobe, along with continued response in the orbital lesion. Additionally, the patient’s tumor markers have remained markedly reduced from baseline, further supporting the sustained radiologic response.
“These encouraging results continue to suggest that our novel Bria-IMT regimen may provide durable immunotherapeutic benefit in late-stage breast cancer patients with brain metastases who have exhausted other options,” stated Dr. William V. Williams, BriaCell’s President & CEO. “The long-term response observed in this patient reinforces the potential of Bria-IMT to improve outcomes while maintaining a favorable tolerability profile.”
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements, including those about BriaCell’s Bria-IMT regimen bringing relief to cancer patients whose medical needs remain unmet and the Bria-IMT regimen becoming a therapeutic option for metastatic breast cancer patients, are based on BriaCell’s current expectations and are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading “Risks and Uncertainties” in the Company’s most recent Management’s Discussion and Analysis, under the heading “Risk Factors” in the Company’s most recent Annual Information Form, and under “Risks and Uncertainties” in the Company’s other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company's profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
info@briacell.com
Investor Relations Contact:
investors@briacell.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/3714a66d-d2af-409d-94df-42bfc0b24842

Figure 1:
Bria-IMT treatment resulted in complete resolution of the right temporal lobe lesion and continued regression of the right orbital (behind the eye) tumor. The right temporal lobe lesion is no longer detectable on the images taken at 8 months (May 2024), 11 months (Aug 2024), and 20 months (May 2025).
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.